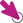What Is THERAKOS® Photopheresis?
The THERAKOS Photopheresis System is an immunomodulatory therapy that uses extracorporeal photopheresis (ECP) to help enhance immunologic response.* THERAKOS Photopheresis was first approved by the FDA in 1987 for the palliative treatment of CTCL skin symptoms in patients who were not responsive to other types of treatment.1,2
Since launch over 35 years ago, there have been over 1 million THERAKOS Photopheresis treatments given. THERAKOS Photopheresis is now available in approximately 170 treatment centers across the United States.3 Treatment centers are independent, third-party facilities not owned, operated, or controlled by Mallinckrodt Pharmaceuticals.
*The exact mechanism of action of UVADEX is not known.
The THERAKOS Photopheresis system includes:
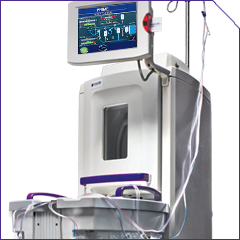
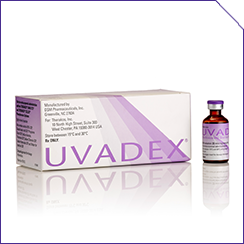
FDA, US Food and Drug Administration.
A Systemic Treatment Option
Skin symptoms of mycosis fungoides, the most common subtype of CTCL, typically have a long, indolent course5,6 Patients may experience a long series of different treatments.
Treatment decisions can incorporate the disease stage, overall prognosis, patient quality of life, and most bothersome symptoms, as well as treatment history and patient preference.7
Some systemic treatment options for CTCL skin manifestations:


When it’s time for systemic therapy, consider one that makes sense for patients in the middle of a long series of possible treatments.5,8
CELLEX Instrument Overview
The CELLEX instrument is a sophisticated touchscreen medical device that is part of an integrated, closed photopheresis system. Only healthcare professionals who have special training and experience in the THERAKOS CELLEX Photopheresis System should operate the CELLEX instrument.4 Healthcare professionals with a background in apheresis are the most common CELLEX operators. Please read the appropriate Operator’s Manual before using this product. To enroll in photopheresis-specific training, visit the MyTHERAKOS portal.
CELLEX automatically calculates the appropriate methoxsalen dose and returns all treated leukocytes to the patient.4
Software upgrades
With these latest upgrades, operators and patients both may benefit from a more automated procedure, as well as a quieter treatment experience with potentially fewer alarms and interruptions.4,9
v5.1 Upgrades |
v5.4 Upgrades |
|---|---|
|
Expanded software automation with potentially Reduced centrifuge speed for a quieter treatment Enhanced prime sequence may result in fewer |
Simplified troubleshooting of some prime Prioritized alarm audio/visual notifications to New operator’s manual includes improved |
Interactive Photo Gallery for CELLEX
Get more information about CELLEX components

References:
-
UVADEX (methoxsalen) Sterile Solution (prescribing information). Therakos Inc; March 2023.
-
Ward DM. Extracorporeal photopheresis: how, when, and why. J Clin Aphersis. 2011;26(5):276-285.
-
Data on File – Ref-VV-00822. Mallinckrodt Pharmaceuticals.
-
THERAKOS® CELLEX® Photopheresis Operator's Manual for Use With Software 5.4. 1470493_Rev06_EN-US. Mallinckrodt; 2020.
-
Prince HM, Whittaker S, Hoppe RT. How I treat mycosis fungoides and Sézary syndrome. Blood. 2009;114(20):4337-4353.
-
Kim EJ, Hess S, Richardson SK, et al. Immunopathogenesis and therapy of cutaneous T-cell lymphoma. J Clin Invest. 2005;115(4):798-812.
-
Lansigan F, Foss FM. Current and emerging treatment strategies for cutaneous T-cell lymphoma. Drugs. 2010;70(3):273-286.
-
Scarisbrick JJ, Bagot M, Ortiz-Romero PI. The changing therapeutic landscape, burden of disease, and unmet needs in patients with cutaneous T-cell lymphoma. Br J Haematol. 2021;192:683-696.
-
Data on File – Ref-VV-01111. Mallinckrodt Pharmaceuticals.
-
THERAKOS® CELLEX® Photopheresis Operator's Manual for Use with Software 5.1. 1470464_RevA-US-UK-CA. Mallinckrodt; 2018.
INDICATIONS AND USAGE
UVADEX® (methoxsalen) Sterile Solution is indicated for extracorporeal administration with the THERAKOS® CELLEX® Photopheresis System in the palliative treatment of the skin manifestations of Cutaneous T-Cell Lymphoma (CTCL) that is unresponsive to other forms of treatment.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
UVADEX is contraindicated in:
-
Patients exhibiting idiosyncratic or hypersensitivity reactions to methoxsalen, other psoralen compounds, or any of the excipients
-
Patients possessing a specific history of a light-sensitive disease state, including lupus erythematosus, porphyria cutanea tarda, erythropoietic protoporphyria, variegate porphyria, xeroderma pigmentosum, and albinism
-
Patients with aphakia because of significantly increased risk of retinal damage
-
Patients that have contraindications to the photopheresis procedure
WARNINGS AND PRECAUTIONS
-
Patients who are receiving concomitant therapy (either topically or systemically) with known photosensitizing agents such as anthralin, coal tar or coal tar derivatives, griseofulvin, phenothiazines, nalidixic acid, halogenated salicylanilides (bacteriostatic soaps), sulfonamides, tetracyclines, thiazides, and certain organic staining dyes such as methylene blue, toluidine blue, rose bengal, and methyl orange may be at greater risk for photosensitivity reactions with UVADEX
-
Oral administration of methoxsalen followed by cutaneous UVA exposure (PUVA therapy) is carcinogenic. Methoxsalen also causes DNA damage, interstrand cross-links and errors in DNA repair
-
Methoxsalen may cause fetal harm when given to a pregnant woman. Women of childbearing potential should be advised to avoid becoming pregnant. If UVADEX is used during pregnancy, or if the patient becomes pregnant while receiving UVADEX, the patient should be apprised of the potential hazard to the fetus
-
Severe photosensitivity can occur in patients treated with UVADEX. Advise patients to wear UVA absorbing, wrap-around sunglasses and cover exposed skin or use a sunblock (SPF 15 or higher), and avoid all exposure to sunlight for twenty-four (24) hours following photopheresis treatment
-
After methoxsalen administration, exposure to sunlight and/or ultraviolet radiation may result in "premature aging" of the skin
-
Since oral psoralens may increase the risk of skin cancers, monitor closely those patients who exhibit multiple basal cell carcinomas or who have a history of basal cell carcinomas
-
Serious burns from either UVA or sunlight (even through window glass) can result if the recommended dosage of methoxsalen is exceeded or precautions are not followed
-
Exposure to large doses of UVA light causes cataracts in animals. Oral methoxsalen exacerbates this toxicity
-
Safety in children has not been established
-
Thromboembolic events, such as pulmonary embolism and deep vein thrombosis, have been reported with UVADEX administration through photopheresis systems for treatment of patients with graft-versus-host disease, a disease for which UVADEX is not approved
ADVERSE REACTIONS
-
Side effects of photopheresis (UVADEX used with the THERAKOS Photopheresis Systems) were primarily related to hypotension secondary to changes in extracorporeal volume (>1%)
INDICATIONS
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS
The THERAKOS CELLEX Photopheresis System is not designated, sold, or intended for use except as indicated.
Certain underlying medical conditions contraindicate THERAKOS Photopheresis, including patients:
-
who cannot tolerate extracorporeal volume loss during the leukocyte-enrichment phase
-
exhibiting idiosyncratic or hypersensitivity reactions to 8-methoxypsoralen/psoralen compounds
-
with coagulation disorders
-
who have had previous splenectomy
WARNINGS AND PRECAUTIONS
-
THERAKOS Photopheresis treatments should always be performed in locations where standard medical emergency equipment is available. Volume replacement fluids and/or volume expanders should be readily available throughout the procedure
-
Patients who may not be able to tolerate the fluid changes associated with extracorporeal photopheresis should be monitored carefully
-
Procedures, such as renal dialysis, which might cause significant fluid changes (and expose the patient to additional anticoagulation) should not be performed on the same day as extracorporeal photopheresis
-
Individual patients may require a heparin dosage that varies from the recommended dose to prevent post-treatment bleeding or clotting during a treatment
ADVERSE REACTIONS
-
Hypotension may occur during any treatment involving extracorporeal circulation. Closely monitor the patient during the entire treatment for hypotension
-
Transient pyretic reactions, 37.7-38.9°C (100-102°F), have been observed in some patients within six to eight hours of reinfusion of the photoactivated leukocyte-enriched blood. A temporary increase in erythroderma may accompany the pyretic reaction
-
Treatment frequency exceeding labeling recommendations may result in anemia
-
Venous access carries a small risk of infection and pain